The woodland has a number of blogs in relation to ‘therapy resistant melancholy’ a variety of subjects from the patient experience to the price effectiveness of interventions akin to long term psychoanalytic psychotherapy.
I put therapy resistant melancholy (TRD) in italics above as chances are you’ll be shocked to know that there isn’t any consensus on its definition, which in flip signifies that the incidence charges fluctuate and there aren’t any constant scientific tips in relation to therapy (Gabriel et al 2023).
The US Meals and Drug Administration and the European Medicines Company have adopted essentially the most used definition of TRD (insufficient response to a minimal of two antidepressants regardless of adequacy of the therapy trial and adherence to therapy). It’s at the moment estimated that not less than 30% of individuals with melancholy meet this definition and so the burden of this side of melancholy is just not insignificant (McIntyre R et al 2023).
The Maudsley Prescribing Tips (Taylor et al 2021) first alternative choices for administration of TRD embody augmentation with lithium and quetiapine. This weblog appears on the new randomised controlled trial by Prof Tony Cleare et al, published today in The Lancet Psychiatry, instantly evaluating the scientific and price effectiveness of the 2. This paper is especially fascinating because the trial (the LQD examine (Lithium versus Quetiapine in Despair)) has a for much longer observe up interval than earlier research, enabling a ‘actual life’ comparability.

The LQD examine, printed right now within the Lancet Psychiatry compares the scientific and price effectiveness of lithium and quetiapine for therapy resistant melancholy.
Strategies
So, this trial is a *takes a deep breath* “part 4, pragmatic, open label, parallel-group, randomised managed superiority trial, evaluating the scientific effectiveness and price effectiveness of lithium versus quetiapine augmentation of antidepressant treatment in contributors with treatment-resistant melancholy.”. Let me break that down for you:
- Section 4: treatment is permitted and being utilized in observe. These trials have a look at long run security and effectiveness in observe
- Pragmatic: trying on the effectiveness of medicines in actual life conditions
- Open label: contributors and researchers know which therapy the contributors are receiving (non-blinded examine)
- Parallel group: two energetic therapy teams, that are then in contrast
- Randomised managed superiority trial: contributors have been randomly assigned to therapy teams and reviewed as to which therapy performs higher.
Medical effectiveness course of: Following random allocation to therapy, trial clinicians may resolve whether or not to proceed with prescription of the allotted treatment based mostly on pre-prescribing security checks and scientific judgement. All contributors, no matter trial treatment standing, have been adopted up over 12 months except they actively withdrew.
The first outcomes have been:
- The Fast Stock of Depressive Symptomatology (QIDS-SR) , used as a weekly measure of temper state and
- Time to discontinuation of therapy.
Weekly knowledge on QIDS-SR, Work and Social Adjustment Scale (WSAS) and trial treatment standing have been collected by way of a web-based platform, True Colors.
Price effectiveness course of: The Shopper Service Receipt Stock was used at baseline, 8, 26, and 52 weeks. This software collects knowledge on health-care service use, together with the quantity and period of contacts with main and secondary health-care providers. High quality-Adjusted Life Years (QALYs) have been used to measure well being advantages.
Outcomes
Over a 4.5 yr interval (Dec 2016 to July 2021) 262 sufferers have been screened for eligibility from 6 NHS Trusts throughout England. The inclusion standards included:
- ≥ 18 years
- Below the care of a GP or psychological well being service
- Present depressive episode assembly DSM-5 standards for main depressive dysfunction (single or recurrent episode)
- Rating of ≥ 14 on the 17 merchandise Hamilton Despair Score Scale
- Insufficient response of the present episode to 2 or extra antidepressants, prescribed for not less than 6 weeks at therapeutic dose
- Present antidepressant therapy unchanged and at therapeutic dose for not less than 6 weeks; and
- Have been capable of present written knowledgeable consent
Exclusion standards included (however not restricted to)
- Analysis of bipolar dysfunction or present psychosis
- Satisfactory use of lithium or quetiapine of their present episode
- Present use of one other atypical antipsychotic.
There have been main, secondary and tertiary outcomes within the examine. For this weblog I’ll deal with the first outcomes and word any key outcomes from the secondary outcomes (tertiary outcomes weren’t included on this publication).
212 sufferers have been randomly assigned:
- 105 assigned to lithium; 21 didn’t obtain or provoke lithium however remained within the trial. 66 offered knowledge at 52 week observe up
- 107 assigned to quetiapine; 12 didn’t obtain quetiapine however remained within the trial. 78 offered knowledge at 52 week observe up
Medical effectiveness outcomes
Main outcomes
- General burden of depressive symptom severity over 12 months
- Time to all trigger discontinuation
Contributors within the quetiapine group had a decrease general burden of depressive symptom severity than contributors within the lithium group over 12 months. The QIDS-SR knowledge factors have been mapped over the yr and the realm below the curve calculation used as a measure of depressive symptom burden. The world below the curve was smaller for Quetiapine: (space below the distinction curve –68.36 [95% CI –129.95 to –6.76]; p=0.0296).
Time to trial treatment discontinuation didn’t differ considerably between the 2 teams; the median time was:
- 365.0 days (Inter-Quartile Vary, IQR 57.0 to 365.0) within the quetiapine group
- 212.0 days (21.0 to 365.0) within the lithium group
- Adjusted hazard ratio [HR] 0.72 [95% CI 0.47 to 1.09]; p=0.1196.
Given the vast IQR and huge discrepancy between the respective medians, we should think about this “absence of proof” relatively than “proof of absence”.
When it comes to secondary outcomes, contributors within the quetiapine group had considerably decrease MADRS (p=0.0435) and WSAS scores (p=0.0071) at week 52 than contributors within the lithium group. No important variations have been famous within the different secondary outcomes which included bodily well being parameters and antagonistic occasions (see paper for full particulars). An fascinating unfavourable end result was that no weight acquire was noticed throughout time within the quetiapine group.
Price effectiveness outcomes
In contrast with lithium, quetiapine was dominant. Prices have been decrease whereas advantages have been increased.
From an NHS and private social providers perspective, quetiapine was related to decrease value and bigger acquire in QALYs, than lithium. The incremental internet well being advantage of Quetiapine was 0.097 over lithium (with any constructive end result indicating choice to the in contrast different). Extra value effectiveness evaluation can be found within the appendices of the paper which define that quetiapine is essentially the most cost-effective choice in response to the NICE willingness-to-pay threshold.
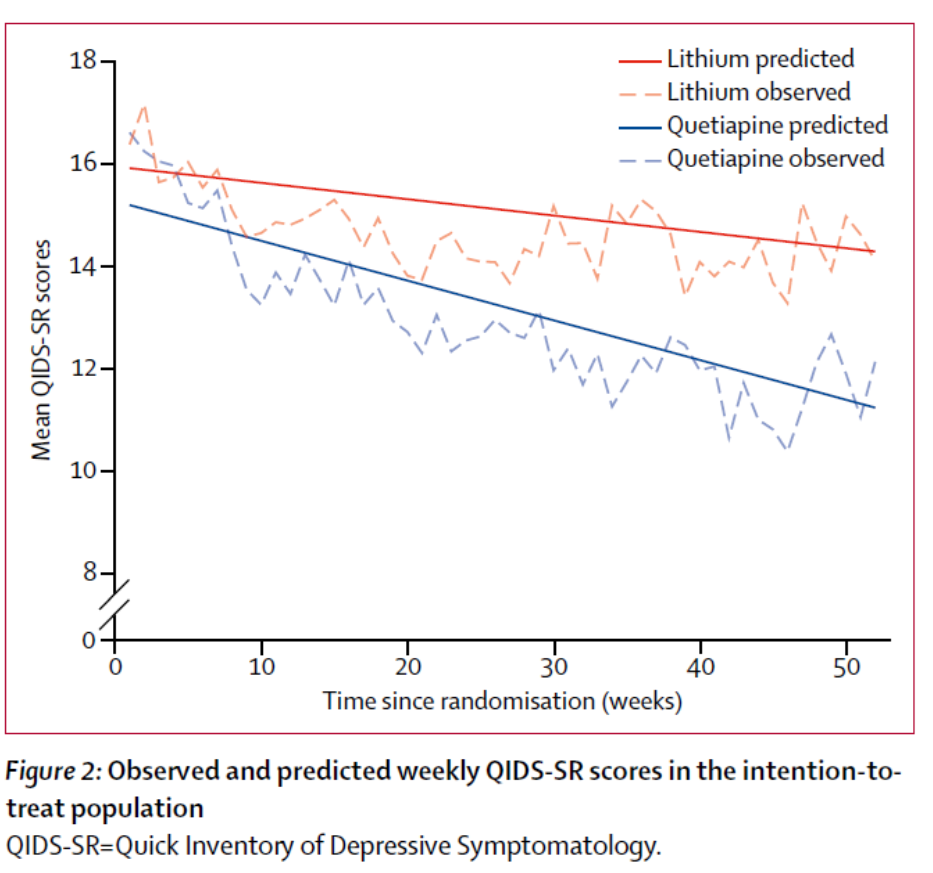
The quetiapine therapy group had a decrease general burden of depressive symptomatology than lithium.
Conclusions
The authors concluded that:
our findings recommend a reasonable and clinically related advantage of quetiapine over lithium for long-term therapy of contributors with treatment-resistant melancholy.
And this examine:
…extends the earlier discovering that quetiapine is non-inferior to lithium over the quick time period and recommend superiority over the long run.

In comparison with lithium, quetiapine is essentially the most cost-effective choice in relation to the NICE willingness-to-pay threshold.
Strengths and limitations
This paper has some actual strengths in that its primary intention was to imitate real-life scientific choices and sufferers. There was lived expertise involvement in designing and operating the trial and affected person and public involvement members have been supportive of the weekly QIDS-SR assessments to offer a greater indication of the course and long-term period of consequence for what generally is a fluctuating scientific course in therapy resistant melancholy (TRD). Following sufferers up for 1 yr was a giant plus.
As a result of nature of the trial, clinicians weren’t blind to allocation, nevertheless the authors report that ‘clinician rated consequence measures have been assessed by masked raters and statisticians have been unaware of group allocation till the information evaluation part’ to attempt to cut back bias as a lot as doable.
With each trial there shall be limitations and this paper is not any exception. Having clinician judgement as as to if the allotted medication is prescribed doubtlessly introduces allocation bias.
The affected person teams have been randomised in response to diploma of therapy resistance (failure of two versus three or extra antidepressant remedies within the present episode) they usually used ‘block randomisation with randomly various block sizes’, nevertheless throughout the outcomes there isn’t any reference as to what number of have been in every group or whether or not the outcomes correlated to this.
Many of the knowledge relied on self-reports. Though this methodology was developed in partnership with affected person teams, the burden could have contributed to attrition.
In the course of the trial, the pattern measurement was decreased from 276 to 214 on account of challenges with recruitment. Energy calculations have been accomplished and have been 80% for time to discontinuation and 96.5% energy with an impact measurement of 0.38. It’s nevertheless unclear if the discontinuation charges, impact sizes and attrition charges have been modified from the unique planning when calculating these. There gave the impression to be doubtlessly regarding gaps within the 52 week assortment knowledge, extra so for lithium (37% for the lithium group and 27% for the Quetiapine group) and the authors word substantial lacking knowledge for among the secondary outcomes.
General, there was a number of attrition, which ought to warning our interpretation of those outcomes. The intention to deal with evaluation solely included 66/104 and 78/107 of the lithium and quetiapine sufferers respectively. The completely different ranges of attrition in every group could imply that we’re not evaluating like with like throughout the teams.
Lastly, the inhabitants examined was predominantly white (89%) which can restrict the flexibility for generalisation to all populations.

Mimicking actual life scientific observe over a yr comes with it’s personal limitations.
Implications for observe
Sufferers who’ve melancholy which is ‘tough to deal with’ are ‘clinically difficult’ and undergo a major burden from the illness. Each lithium and quetiapine are widespread choices for augmentation and this paper highlights that quetiapine may very well be extra efficacious and price efficient than lithium. The size of observe up of the examine makes this encouraging and definitely value contemplating. The ability of the examine and the relatively heterogeneous group of severity could restrict leaping to a direct use of quetiapine over lithium, but when there was future replication of this examine and outcomes, then that will surely be convincing.
The authors are clear although that Lithium stays an efficient therapy choice. It’s probably that the medicines may have completely different advantages for various individuals (e.g. issues in relation to sleep, urge for food, anxiousness) and so therapy needs to be tailor-made to those wants. Nevertheless, if lithium and quetiapine are equally acceptable then quetiapine may pip lithium to the publish.
Having clear scientific tips in relation to methods for ‘tough to deal with’ melancholy and/or when it turns into ‘therapy resistant’, appears a precedence in order that future analysis might be evaluating apples with apples.
On the time of scripting this, there’s a complicating think about that there’s a nationwide scarcity of modified launch quetiapine and we’re having to maneuver sufferers onto rapid launch Quetiapine which has a special facet impact profile and should not produce outcomes replicable to the examine.

Quetiapine could pip lithium to the publish if on a good area.
The non-public influence of therapy resistant melancholy can’t be underestimated and I’m positive they might agree with the phrases of Bon Jovi ‘I simply need to reside whereas I’m alive…it’s my life’.
Assertion of pursuits
I’ve no battle of pursuits to reveal
Hyperlinks
Main paper
Anthony J Cleare, Jess Kerr-Gaffney, Kimberley Goldsmith, Zohra Zenasni, Nahel Yaziji, Huajie Jin, Alessandro Colasanti, John R Geddes, David Kessler, R Hamish McAllister-Williams, Allan H Younger, Alvaro Barrera, Lindsey Marwood, Rachael W Taylor, Helena Tee, and on behalf of he LQD Research Group. (2025) Clinical and cost-effectiveness of lithium versus quetiapine augmentation for treatment-resistant depression in England: a pragmatic, open-label, parallel-group, randomised controlled superiority trial. The Lancet Psychiatry 2025. DOI: 10.1016/S2215-0366(25)00028-8
Different references
Gabriel FC et al (2023) Guidelines’ recommendations for the treatment-resistant depression: A systematic review of their quality. PLoS ONE 18(2): e0281501.
McIntyre RS, et al (2023) Treatment-resistant depression: definition, prevalence, detection, management, and investigational interventions. World Psychiatry. (2023) 22, no. 3, 394–412.
Taylor, David M, et al. The Maudsley Prescribing Tips in Psychiatry. 14th ed., John Wiley & Sons, 2021 pg 318-319.
